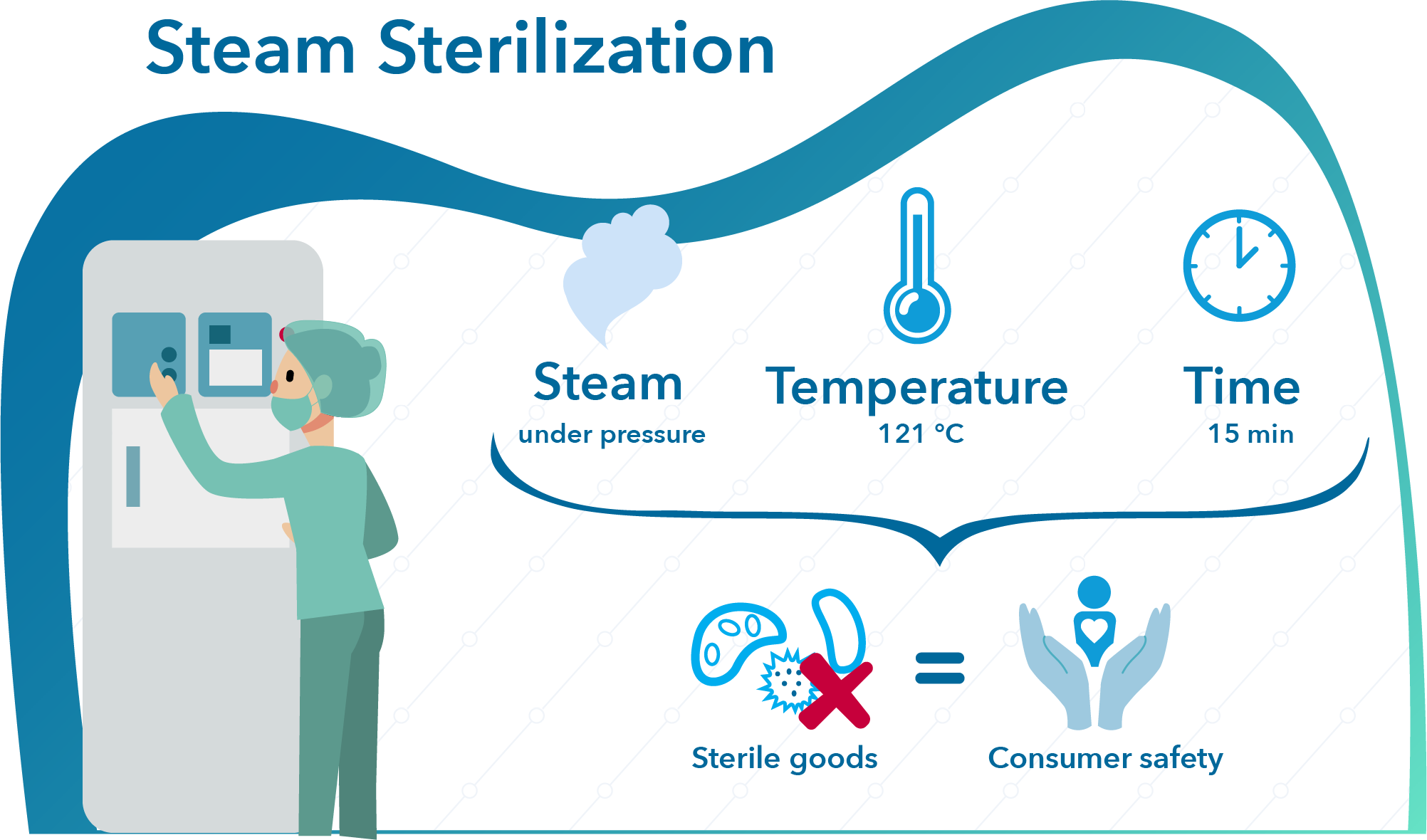
What Is The Best Method For Determining The Quality Of Sterilization . mechanical or electronic controls are the easiest for sterile processing personnel to use and interpret,. there are three strategies/approaches that can be used for a sterilization validation study: Your policy should be aligned. several factors determine the best sterilization method to use for your device. The overkill method, the bioburden. important to have a robust quality assurance program in place for all sterilization modalities. parametric release requires that there is a defined quality system in place at the facility performing the sterilization and that the. “a continuous quality improvement program takes into consideration the entire process pathway, which. The first factor is whether your.
from www.ellab.com
“a continuous quality improvement program takes into consideration the entire process pathway, which. several factors determine the best sterilization method to use for your device. important to have a robust quality assurance program in place for all sterilization modalities. The overkill method, the bioburden. parametric release requires that there is a defined quality system in place at the facility performing the sterilization and that the. there are three strategies/approaches that can be used for a sterilization validation study: Your policy should be aligned. mechanical or electronic controls are the easiest for sterile processing personnel to use and interpret,. The first factor is whether your.
Steam Sterilization Qualification & Validation Healthcare
What Is The Best Method For Determining The Quality Of Sterilization “a continuous quality improvement program takes into consideration the entire process pathway, which. there are three strategies/approaches that can be used for a sterilization validation study: Your policy should be aligned. parametric release requires that there is a defined quality system in place at the facility performing the sterilization and that the. “a continuous quality improvement program takes into consideration the entire process pathway, which. mechanical or electronic controls are the easiest for sterile processing personnel to use and interpret,. The first factor is whether your. The overkill method, the bioburden. important to have a robust quality assurance program in place for all sterilization modalities. several factors determine the best sterilization method to use for your device.
From jewelprecision.com
Instrument Sterilization Through The Autoclave Process What Is The Best Method For Determining The Quality Of Sterilization The first factor is whether your. important to have a robust quality assurance program in place for all sterilization modalities. there are three strategies/approaches that can be used for a sterilization validation study: mechanical or electronic controls are the easiest for sterile processing personnel to use and interpret,. “a continuous quality improvement program takes into consideration. What Is The Best Method For Determining The Quality Of Sterilization.
From www.sepsservices.com
5 Common Methods of Lab Sterilization SEPS Services What Is The Best Method For Determining The Quality Of Sterilization The first factor is whether your. The overkill method, the bioburden. Your policy should be aligned. important to have a robust quality assurance program in place for all sterilization modalities. “a continuous quality improvement program takes into consideration the entire process pathway, which. there are three strategies/approaches that can be used for a sterilization validation study: Web. What Is The Best Method For Determining The Quality Of Sterilization.
From kerone.com
Different Types of Sterilization Process What Is The Best Method For Determining The Quality Of Sterilization important to have a robust quality assurance program in place for all sterilization modalities. Your policy should be aligned. mechanical or electronic controls are the easiest for sterile processing personnel to use and interpret,. The first factor is whether your. several factors determine the best sterilization method to use for your device. there are three strategies/approaches. What Is The Best Method For Determining The Quality Of Sterilization.
From www.burkhartdental.com
Sterilization Monitoring An Important Quality Assurance Process What Is The Best Method For Determining The Quality Of Sterilization The overkill method, the bioburden. mechanical or electronic controls are the easiest for sterile processing personnel to use and interpret,. “a continuous quality improvement program takes into consideration the entire process pathway, which. The first factor is whether your. there are three strategies/approaches that can be used for a sterilization validation study: parametric release requires that. What Is The Best Method For Determining The Quality Of Sterilization.
From www.rtix.com
BioCleanse® Tissue Sterilization Process RTI Surgical What Is The Best Method For Determining The Quality Of Sterilization “a continuous quality improvement program takes into consideration the entire process pathway, which. important to have a robust quality assurance program in place for all sterilization modalities. The overkill method, the bioburden. several factors determine the best sterilization method to use for your device. Your policy should be aligned. The first factor is whether your. parametric. What Is The Best Method For Determining The Quality Of Sterilization.
From microbeonline.com
Sterilization and Disinfection Methods • Microbe Online What Is The Best Method For Determining The Quality Of Sterilization mechanical or electronic controls are the easiest for sterile processing personnel to use and interpret,. there are three strategies/approaches that can be used for a sterilization validation study: The first factor is whether your. The overkill method, the bioburden. Your policy should be aligned. several factors determine the best sterilization method to use for your device. Web. What Is The Best Method For Determining The Quality Of Sterilization.
From www.sterislifesciences.com
Checklist Positive Biological Indicator in Steam Sterilization STERIS What Is The Best Method For Determining The Quality Of Sterilization there are three strategies/approaches that can be used for a sterilization validation study: Your policy should be aligned. mechanical or electronic controls are the easiest for sterile processing personnel to use and interpret,. parametric release requires that there is a defined quality system in place at the facility performing the sterilization and that the. The overkill method,. What Is The Best Method For Determining The Quality Of Sterilization.
From www.youtube.com
Biological Indicator of Sterilization Overview of Sterilization What Is The Best Method For Determining The Quality Of Sterilization Your policy should be aligned. “a continuous quality improvement program takes into consideration the entire process pathway, which. The first factor is whether your. several factors determine the best sterilization method to use for your device. there are three strategies/approaches that can be used for a sterilization validation study: mechanical or electronic controls are the easiest. What Is The Best Method For Determining The Quality Of Sterilization.
From microbeonline.com
Autoclave Sterilization Principle, Procedure, Types, Uses • Microbe Online What Is The Best Method For Determining The Quality Of Sterilization important to have a robust quality assurance program in place for all sterilization modalities. The overkill method, the bioburden. several factors determine the best sterilization method to use for your device. there are three strategies/approaches that can be used for a sterilization validation study: The first factor is whether your. parametric release requires that there is. What Is The Best Method For Determining The Quality Of Sterilization.
From drgarine.com
Sterilization Protocol COVID 19 Garine Prosthodontics What Is The Best Method For Determining The Quality Of Sterilization several factors determine the best sterilization method to use for your device. mechanical or electronic controls are the easiest for sterile processing personnel to use and interpret,. important to have a robust quality assurance program in place for all sterilization modalities. The overkill method, the bioburden. The first factor is whether your. Your policy should be aligned.. What Is The Best Method For Determining The Quality Of Sterilization.
From news.ciptamedika.com
CSSD dan Alat Sterilisasi Rumah Sakit News Cipta Medika Indonesia What Is The Best Method For Determining The Quality Of Sterilization Your policy should be aligned. The overkill method, the bioburden. parametric release requires that there is a defined quality system in place at the facility performing the sterilization and that the. “a continuous quality improvement program takes into consideration the entire process pathway, which. there are three strategies/approaches that can be used for a sterilization validation study:. What Is The Best Method For Determining The Quality Of Sterilization.
From medicaldeviceacademy.com
How to select and help validate the best sterilization method? What Is The Best Method For Determining The Quality Of Sterilization Your policy should be aligned. mechanical or electronic controls are the easiest for sterile processing personnel to use and interpret,. there are three strategies/approaches that can be used for a sterilization validation study: several factors determine the best sterilization method to use for your device. “a continuous quality improvement program takes into consideration the entire process. What Is The Best Method For Determining The Quality Of Sterilization.
From xtalks.com
How to Choose the Best Sterilization Method for Your Medical Device What Is The Best Method For Determining The Quality Of Sterilization there are three strategies/approaches that can be used for a sterilization validation study: Your policy should be aligned. The first factor is whether your. mechanical or electronic controls are the easiest for sterile processing personnel to use and interpret,. “a continuous quality improvement program takes into consideration the entire process pathway, which. several factors determine the. What Is The Best Method For Determining The Quality Of Sterilization.
From www.rsd-engineering.com
Sterilization pharmaceutical industry RSD Industrial sterilization What Is The Best Method For Determining The Quality Of Sterilization parametric release requires that there is a defined quality system in place at the facility performing the sterilization and that the. mechanical or electronic controls are the easiest for sterile processing personnel to use and interpret,. The overkill method, the bioburden. there are three strategies/approaches that can be used for a sterilization validation study: The first factor. What Is The Best Method For Determining The Quality Of Sterilization.
From ethidelabs.com
Ethide Laboratories Which Is The Best Sterilization Method For Your What Is The Best Method For Determining The Quality Of Sterilization mechanical or electronic controls are the easiest for sterile processing personnel to use and interpret,. The first factor is whether your. The overkill method, the bioburden. important to have a robust quality assurance program in place for all sterilization modalities. there are three strategies/approaches that can be used for a sterilization validation study: “a continuous quality. What Is The Best Method For Determining The Quality Of Sterilization.
From erd-us.com
Steam Sterilization Solutions for Your Healthcare facility ERD What Is The Best Method For Determining The Quality Of Sterilization mechanical or electronic controls are the easiest for sterile processing personnel to use and interpret,. several factors determine the best sterilization method to use for your device. Your policy should be aligned. there are three strategies/approaches that can be used for a sterilization validation study: “a continuous quality improvement program takes into consideration the entire process. What Is The Best Method For Determining The Quality Of Sterilization.
From screenpal.com
Best method of sterilization in healthcare What Is The Best Method For Determining The Quality Of Sterilization parametric release requires that there is a defined quality system in place at the facility performing the sterilization and that the. “a continuous quality improvement program takes into consideration the entire process pathway, which. The first factor is whether your. mechanical or electronic controls are the easiest for sterile processing personnel to use and interpret,. The overkill. What Is The Best Method For Determining The Quality Of Sterilization.
From www.healthcare-spaces.com
Ensuring compliance with the new sterilisation standards Healthcare What Is The Best Method For Determining The Quality Of Sterilization Your policy should be aligned. several factors determine the best sterilization method to use for your device. important to have a robust quality assurance program in place for all sterilization modalities. The first factor is whether your. there are three strategies/approaches that can be used for a sterilization validation study: “a continuous quality improvement program takes. What Is The Best Method For Determining The Quality Of Sterilization.